The mass number is unchanged and the atomic number decreases. Transmutation converting to another nuclide.
Transmutation converting to another nuclide.
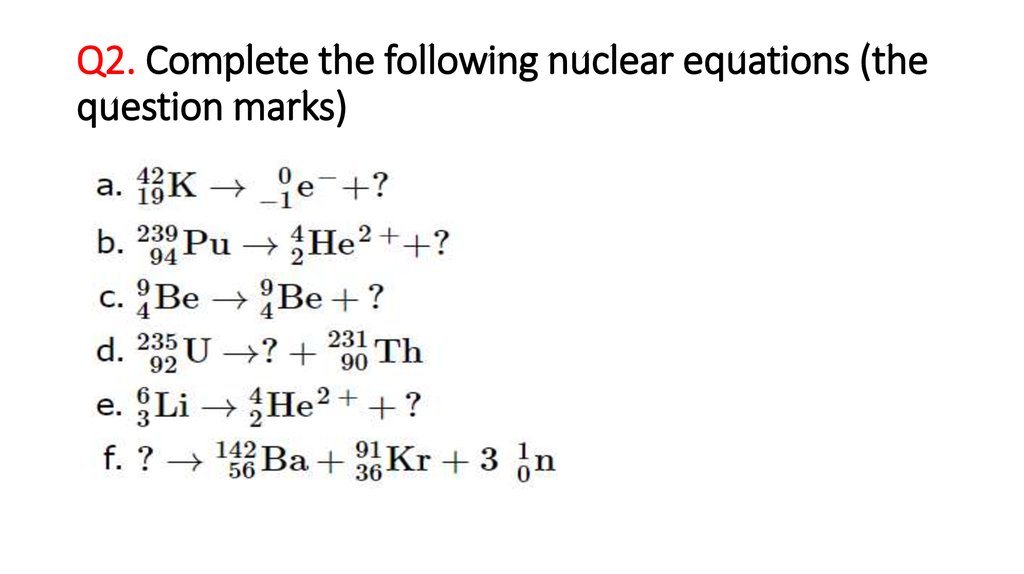
Complete the following nuclear reaction. Complete the following nuclear reaction equations. In nuclear decay the nuclear reaction holds the conservation of mass number and conservation of atomic number. In a nuclear reaction the atomic numbers and mass numbers are balanced.
Solution for Complete the following nuclear reaction equation. Co Mn He 56 27 56 25 a u e O b 0 1 O d ge e He. Complete the following nuclear equations and identify X in each case.
View Answer Identify X in the following nuclear reaction a 1H 9Be X n. B 12C 1H X. C 15N 1H 4He X.
View Answer Complete the following nuclear-decay equations by filling in the blanks. Asked Oct 6 2018 in Physics by Minu 460k points retagged Oct 6 2018 by Minu. Complete the following nuclear reactions.
A 10 5 B 01 n 24He. B 9442Mo 21n. Click hereto get an answer to your question Complete the following nuclear reaction.
Complete the following nuclear reaction. 2 pts - 92 U 4-2He 231 - Th. See the answer.
See the answer See the answer done loading. Complete the following nuclear reaction. Complete the following nuclear reaction.
2 pts - 92 U 4-2He 231 - Th. Changes of nuclei that result in changes in their atomic numbers mass numbers or energy states are nuclear reactions. To describe a nuclear reaction we use an equation that identifies the nuclides involved in the reaction their mass numbers and atomic numbers and the other particles involved in the reaction.
Types of Particles in Nuclear Reactions. Many entities can be involved in nuclear reactions. Complete the following nuclear reactions.
A 714N_24He rarr 817O___. B _1530P rarr _1430Si_____. Calculate dispersive power of glass accurately up to decimal places from the following data.
Refractive index of glass for red colour 160. Refractive index of glass for yellow colour 161. Refractive index of glass for violet colour 162.
Complete the following nuclear reactions. I _7_14N _24He rarr _8O _11H ii 24Mg _24He rarr _1327Al ii. Nuclear decay fill in the blanks to complete the following nuclear reactions.
Transmutation converting to another nuclide. Each radioactive nuclide has its own half life. Answer to Complete the following nuclear reactionsa.
Laura is a single taxpayer living in New Jersey with adjusted gross income for the 2019 tax year of 35550. Lauras employer withheld 3410 in state income tax from her salary. Complete the following nuclear reactions and identify the type of nuclear reaction pha decay beta decay Type of Nuclear Reaction Alpha Decay 78175PT 89228AC - 10e 92238U 90234Th 919F 24HE 86210RN 24HE close.
Start your trial now. First week only 499. Calculate the for the reaction if it fissions evenly into nuclei and emits neutrons.
Use the equation for atomic mass to answer the following questions. Nuclear reactions atomic structure radioactive decay energy changes and. Fill in the blanks to complete the following nuclear reactions.
Complete the following nuclear reactions. 226 z 26 b. 4 He alpha decay alpha decay bea decay When isotope bismuth-213 emits an alpha particle.
Write out the nuclear equation. 213 zoa 83 Bi 213 b. Which is the parent element.
Which is the daughter element. The reaction in our example above would be written as Li-6dαα. Balancing a Radioactive Decay Equation.
In balancing a nuclear equation it is important to remember that the sum of all the mass numbers and atomic numbers given on the upper left and lower left side of the element symbol respectively must be equal for both sides of the equation. The following reaction represents what nuclear process. Complete the following equation of nuclear transmutation.
Describe what changes occur during electron capture. The mass number is unchanged and the atomic number decreases. The energy released from the sun is the result of a nuclear fission reaction.
Water is used to moderate slow down neutrons in a nuclear reactor. Which of the following naturally occurring radioisotopes would be most useful in dating objects thought to be millions of years old. T12 573 x 103 years.
That is released and theres three of them on this actually Really particularly or in reaction. This is the breakdown of uranium for your nuclear power plants. Listen important reaction here.
Atomic number 24 plus two equals zero plus acts. And so we could see acts of you is gonna be 26 atomic number the mast on end mass and be 53. Supply the missing atomic symbol to complete the equation for the following nuclear fission reaction.
Also how do you decipher whether its 2 10n rather than 3. The lower numbers must add to the same on both sides. The upper numbers must add to the same on both sides.
You have 92 0 on the left. There must be 92 on the right. Changes of nuclei that result in changes in their atomic numbers mass numbers or energy states are nuclear reactions.
To describe a nuclear reaction we use an equation that identifies the nuclides involved in the reaction their mass numbers and atomic numbers and the other particles involved in the reaction. Types of Particles in Nuclear Reactions. Many entities can be involved in nuclear reactions.
Write a balanced equation for each of the following nuclear reactions. A bismuth-212 decays into polonium-212 b beryllium-8 and a positron are produced by the decay of an unstable nucleus c neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239 d strontium-90 decays into yttrium-90.