
Describe the role of energy in chemical reactions. Energy is used to break bonds in reactants and energy is released when new bonds form in products.
Energy is used to break bonds in reactants and energy is released when new bonds form in products.
Describe the role of energy in chemical reactions. All chemical reactions involve energy. Energy is used to break bonds in reactants and energy is released when new bonds form in products. Like the combustion reaction in a furnace some chemical reactions require less energy to break bonds in reactants than is released when bonds form in products.
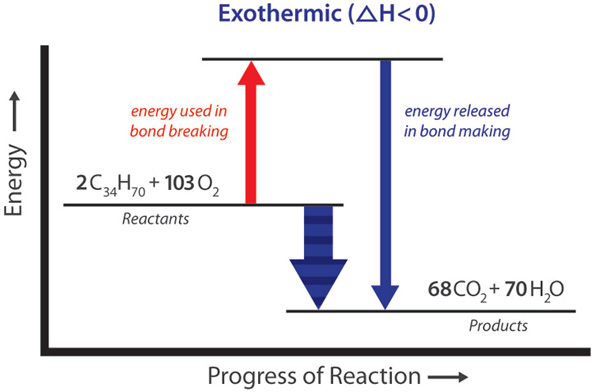
These reactions called exothermic reactions release energy. Energy plays a key role in chemical processes. According to the modern view of chemical reactions bonds between atoms in the reactants must be broken and the atoms or pieces of molecules are reassembled into products by forming new bonds.
Energy is absorbed to break bonds and energy is evolved as bonds are made. Energy is central to chemical reactions. Some release it while others require energy to be added before they can take place.
Understanding the role of energy in chemistry provides insight into all kinds of questions about daily life. The energy change in a chemical reaction is due to the difference in the amounts of stored chemical energy between the products and the reactants. This stored chemical energy or heat content of the system is known as its enthalpy.
Exothermic reactions release heat and light into their surroundings. Describe the role of energy in chemical reactions. Some chemical reactions release energy and other chemical reactions absorb energy.
Energy changes determine how easily a chemical reaction will occur. The Role of Energy in Chemical Reactions Chemical reactions require a sufficient amount of energy to cause the matter to collide with enough precision and force that old chemical bonds can be broken and new ones formed. The term used to describe all of the chemical reactions inside a cell is metabolism Figure 82.
Cellular processes such as the building or breaking down of complex molecules occur through series of stepwise interconnected chemical reactions called metabolic pathway s. Reactions that are spontaneous and release energy are exergonic reactions whereas endergonic reactions require. Because catabolic reactions produce energy and anabolic reactions use energy ideally energy usage would balance the energy produced.
If the net energy change is positive catabolic reactions release more energy than the anabolic reactions use then the body stores the excess energy by building fat molecules for long-term storage. In some reactions the energy that must be absorbed to break the bonds in the reactants is less than the energy that is released when the new bonds of the products are formed. This means that in the overall reaction energy is released as either heat or light.
All chemical reactions need something that makes them start going. Chemical reactions will not take place until the system has some minimum amount of energy added to it. This energy is called the activation energy.
Activation energy is the minimum amount of energy that is needed to start a chemical reaction. 69 Critical Thinking Questions. Describe the connection between anabolic and catabolic chemical reactions in a metabolic pathway.
Catabolic reactions produce energy and simpler compounds whereas anabolic reactions involve the use of energy to make more complex compounds. Catabolic reactions produce energy and complex compounds are formed. The activation energy of a chemical reactions is the minimum amount of energy needed for the reactants involved in the reaction to form the product.
A catalyst such as an enzyme is a substance that lowers the activation energy needed to start the reaction. Scientists use the term bioenergetics to describe the concept of energy flow through living systems such as cellsCellular processes such as the building and breaking down of complex molecules occur through stepwise chemical reactionsSome of these chemical reactions are spontaneous and release energy whereas others require energy to proceed. Energy is needed to perform heavy labor and exercise but humans also use a great deal of energy while thinking and even while sleeping.
For every action that requires energy many chemical reactions take place to provide chemical energy to the systems of the. Food is rearranged through chemical reactions forming new molecules that release energy as this matter moves through an organism. Molecules are broken apart and put back together to form new substances and in this process energy is released.
Cellular respiration in plants and animals involves chemical reactions with. In essence the ATP is used in various chemical reactions that need energy as a booster for these reactions. In organisms energy coupling is typically shown based on ATP production and hydrolysis.
Catabolic reactions generate the ATP while the ATP produced drives forward the. ATP plays an essential role in the transfer of free energy from energy-yielding to energy-requiring processes of the cells. This ATP breaks down into ADP Adenosine diphosphate and Pi inorganic phosphate there by donating its chemical energy to carry out the various functions like biosynthesis of macromolecules transport of ions molecules and for doing mechanical work.